It contains a complete solute. 2 There is equal movement of sodium and potassium between intracellular and extracellular fluids.

Elektrochemische Zellen Elektrochemische Zellen Teaching Chemistry Electrochemistry Science Chemistry
Contains a completely dissociated solute Choose.

. Is a strong electrolyte as it completely dissociates into Ca 2 and NO 3. An electrolyte is a medium containing ions that is electrically conducting through the movement of ions but not conducting electrons. Does not dissociate in solution.
List of Electrolytes 1. The sodium is the solute C. 1 There is a greater concentration of sodium in extracellular fluid and a greater concentration of potassium in intracellular fluid.
Correct option is D A strong electrolyte will completely dissociate into its component ions in solution. Which of the following statements is true concerning the sodium. 2 on a question.
An electrolyte is a substance that produces an electrically conducting solution when dissolved in a polar solvent such as water. Which statement describes an electrolyte. Solid-state electrolytes also exist.
Upon dissolving the substance separates into cations and anions which disperse uniformly throughout the solvent. Oxalic acid carbonic acid NH 4. The process in which neutral molecules lose or gain electrons.
Determine whether each statement describes a solution of a strong electrolyte weak electrolyte or non-electrolyte. A strong acid is any acid that ionizes completely in solutions. Chemistry questions and answers.
Which of the following statements best describes electrolytes in extracellular and intracellular fluid. Solutions for Chapter 25 Problem 6DYB. Solution for Which of the following solutions of strong electrolytes contains the largest number of moles of chloride ions.
10 Questions Show answers. The mixing of different substances. What is the concentration of oh ions in the solution.
An electrolyte conducts an electric current as a solid and does not dissolve in water. When humans sweat we lose ions necessary for vital bodily functions. The sodium is the solution B.
When an electrolyte dissolves in water the resulting solution conducts an electric current. Does not dissociate in solutiond. The dissolved electrolyte separates into wo kinds of ions.
A Pb2 b ClO3- c Na d I-. This includes most soluble salts acids and bases dissolved in a polar solvent such as water. Does not conduct an electric currentb.
Has a medium level of conductivity Choose. If an electrical current can run through a solution it is said to contain. The sodium is the solvent D.
These substances constitute an important class of compounds called electrolytesSubstances that do not yield ions when dissolved are called nonelectrolytesIf the physical or chemical process that generates the ions is essentially 100. 1000 mL of 030 M AlCl3 500 mL. Is covalently bonded and usually contains carbonc.
Does not conduct an electric current. It contains a partially dissociated solute. Which of the following describes an electrolyte.
Plays a significant role in regulating fluid balance. Which of the following is an electrolyte. To replenish them we need to consume more ions often in the form of an electrolyte solution.
For each write a balanced equation for their dissociation in water. In the human body electrolytes have. In the reaction between aqueous solutions of leadII chlorate and sodium iodide which ions become spectator ions.
Gatorade as an electrolyte solution The sports drink Gatorade advertises that it contains electrolytes because it contains sodium potassium magnesium and other ions. Correct option is A A solute solution that completely ionizes or dissociates in a solution is called as strong electrolyte. Which of the following describes an electrolyte.
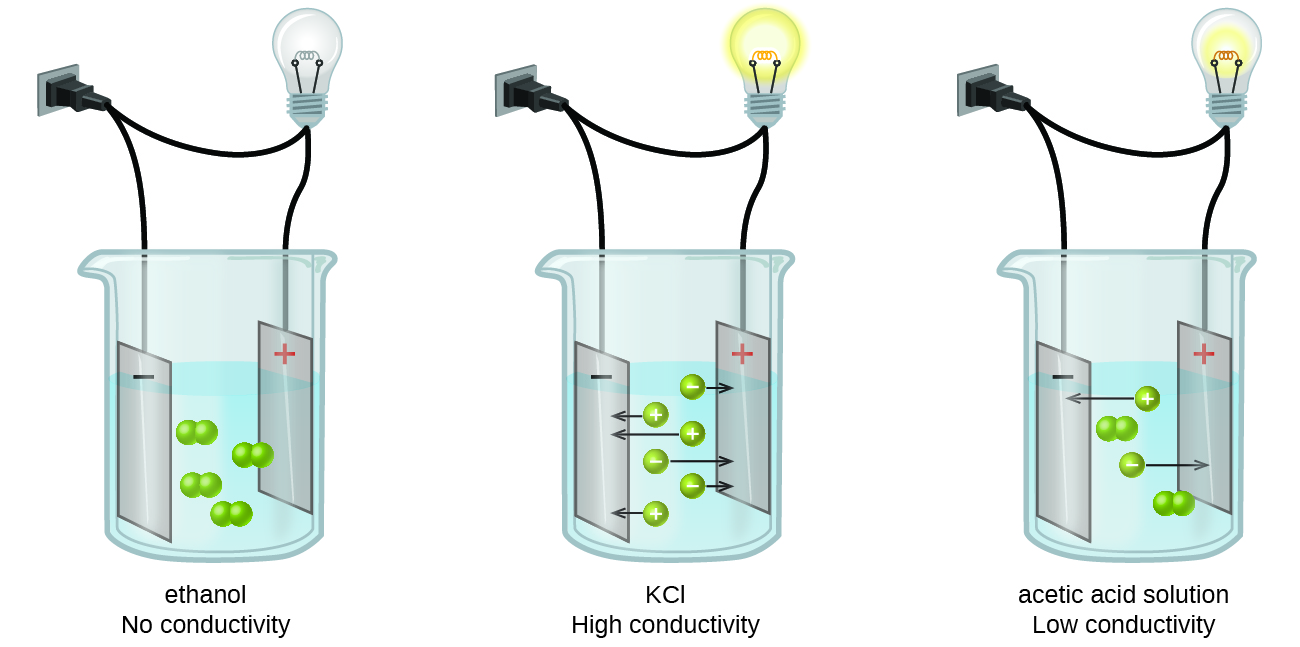
Is covalently bonded and usually contains. Important electrolytes other than sodium and chloride include potassium calcium bicarbonate and phosphate. When the ends of two wires from a circuit containing a battery and a lightbulb are placed into a beaker containing an aqueous solution the light bulb glows brightly.
And sodium acetate are weak electrolytes. Is covalently bonded and usually contains. Has little or no conductivity Choose.
This means it gives off the greatest number of hydrogen ions or protons when placed in a solution. All of the above are true concerning the sodium. Does not conduct an electric current.
When some substances are dissolved in water they undergo either a physical or a chemical change that yields ions in solution. It contains a completely dissociated solute. Has the highest conductivity Choose.
It has the highest conductivity. Such sport drinks commonly contain 4 to 8 carbohydrate as glucose fructose sucrose or maltodextrins and small amounts of electrolytes most often sodium potassium and chloride. Ions in an aqueous solution.
An electrolyte is simply seen as any substance that conducts electricity when dissolved in water. Answer of Which of the following describes an electrolyte. Is covalently bonded and usually contains carbon.
Major Electrolytes Outside the Cell. An electrolyte conducts an electric current as a solid and dissolves in water. Plays a significant role in regulating fluid balance.
A weak electrolyte will remain mostly undissociated in solution and dissociates very less. The following soluble salts are strong electrolytes. Sodium and chloride the major electrolytes in extracellular fluid exert most of their influence outside the cell.
Cations and anions which disperse uniformly through the solvent. Helpful 0 Not. What is the molarity of a solution containing 70 moles of solute in 569 ml of solution What is the molarity of a solution that contains 6 moles of solute in 2 liters of solution The poh of a solution is 1075.
Solutions in which electric currents cannot run. It has a medium level of conductivity. Weak electrolyte solution.
Does not conduct an electric current. A variety of beverages formulated to provide fluid carbohydrates and electrolytes during and following exercise are commercially available. An IV solution contains the electrolyte sodium.

Solutes Electrolytes And Non Electrolytes Ppt Download

Physical Chemistry Positive Or Negative Anode Cathode In Electrolytic Galvanic Cell Chemistry Stack Exch Electrochemistry Chemistry Classroom Galvanic Cell

0 Comments